Pregnancy
Pregnancy is a special time for the entire expecting family. It is also an important time to take steps to help keep yourself and your baby protected from vaccine-preventable diseases. The vaccines that are recommended during pregnancy protect you and baby now and throughout your child’s life.
Before Becoming Pregnant
If you are planning to become pregnant, now is an important time to check that you are up to date on all recommended vaccines. Some vaccines are especially important for women who are planning to become pregnant because the diseases they protect against can be especially harmful if you get sick during your pregnancy (like measles and rubella). If you can plan ahead, check in on your vaccination status. Learn more about the vaccines that can help you prepare for a healthy pregnancy.
Vaccines During Pregnancy
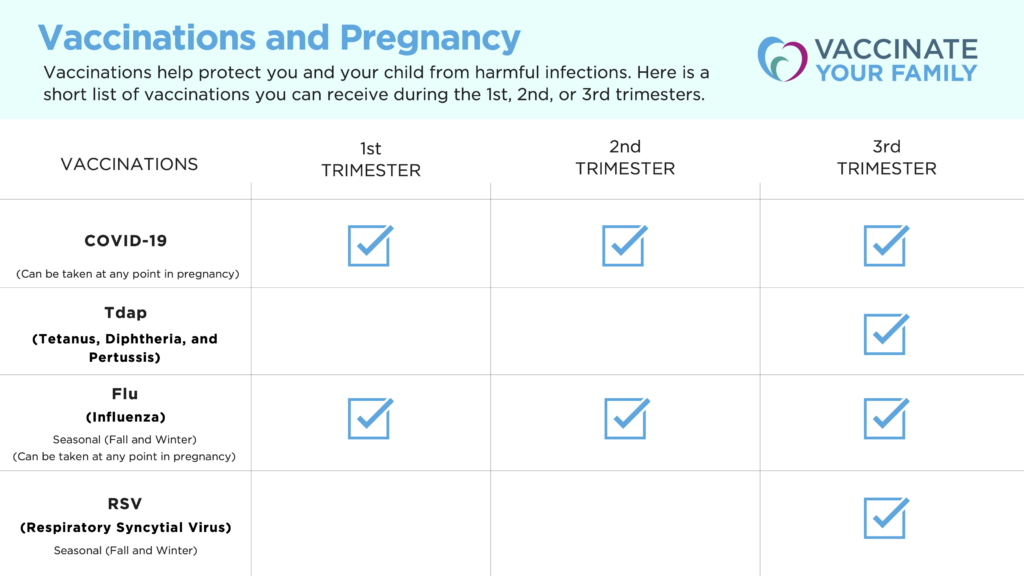
Did you know you can protect your baby from disease during pregnancy? Getting vaccinated while you are pregnant cues your body to create protective antibodies that you can pass on to your baby. These antibodies can help protect your baby during their early months of life, before they’re able to get vaccinated themselves.
COVID-19 Vaccine
The CDC strongly recommends pregnant and breastfeeding individuals get vaccinated against COVID-19. Why? If you are pregnant, you are more likely to get very sick from COVID-19. Getting the COVID-19 vaccine can help protect both you and your baby from serious illness from COVID-19. For answers to your COVID-19 vaccine questions, visit VYF’s Questions and Answers about COVID-19 Vaccines.
Tetanus, Diphtheria, and Pertussis (Tdap) Vaccine
The Tdap (Tetanus, Diphtheria, and Pertussis) vaccine helps protect against whooping cough which can be very dangerous for your baby. To best protect both you and your baby from whooping cough, the CDC recommends you take the Tdap vaccine during the early part of your 3rd trimester for each pregnancy. If you cannot get the vaccine during your pregnancy, get it immediately after giving birth so some antibodies against whooping cough can pass to your baby if you are breastfeeding.
Influenza (Flu) Vaccine
Influenza (Flu) is more likely to cause hospitalizations and deaths among pregnant people and can be harmful to your developing baby. Getting the flu vaccine can protect the pregnant person from flu or flu-related hospitalizations and prevent complications in labor and delivery. The CDC recommends taking the flu shot during any trimester to best protect both you and your baby. Learn more about this flu season.
Respiratory Syncytial Virus (RSV) Vaccine
RSV is a lower respiratory virus and has become the leading cause of hospitalizations in infants in the United States. As of September 22, 2023, CDC recommends a RSV (Respiratory Syncytial Virus) vaccine during weeks 32-36 (3rd trimester) of pregnancy to protect your baby from severe RSV.
Public health and pregnancy experts strongly support getting vaccinated during pregnancy, groups making this recommendation include: Centers for Disease Control and Prevention (CDC), the American College of Obstetricians and Gynecologists (ACOG), and the American College of Nurse-Midwives.
Pregnancy is also good time to start thinking about the vaccines your baby will need once they are born. Visit our Babies & Children section to learn more about the importance of vaccinating your child according to the recommended immunization schedule.
Commonly Asked Questions About Vaccines for Pregnant People
Is the flu vaccine safe for me and unborn baby? I heard something about the flu vaccine causing miscarriages.
Yes. The CDC, the American Academy of Family Physicians (AAFP), the American College of Nurse-Midwives (ACNM), the American College of Obstetricians and Gynecologists(ACOG) ,the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN) and other pregnancy experts all strongly recommend that pregnant people get a flu shot for the best protection – for them and their babies – against serious flu illness and flu-related complications.
The flu shot is safe, during any trimester, for both you and your baby. The flu shot has been safely given to millions of pregnant individuals over many years. You can not get the flu – or give your baby the flu – from getting the flu vaccine.
Multiple studies have shown that people who have gotten flu shots during pregnancy HAVE NOT had a higher risk for miscarriage. One of the largest and strongest studies was conducted in CDC’s Vaccine Safety Datalink (VSD) project. The study looked at three flu seasons to see if there was any increased risk for miscarriage among pregnant women who had received a flu vaccine during their pregnancy. The study found NO increased risk for miscarriage after flu vaccination during pregnancy
It is also safe for women to get the flu vaccine while breastfeeding. In fact, breastfeeding also helps to protect babies because breast milk passes your antibodies to your baby, and these antibodies help your baby fight off flu.
Following is a list of studies that show that the flu vaccine is safe during pregnancy. Click on the studies below to read the research.
- Review of reports to the Vaccine Adverse Reporting System (VAERS) (Moro et al, 2011, Moro et al, 2011 Moro et al, 2017) – A review of the reports to VAERS found no evidence to suggest a link between pregnancy complications or adverse fetal outcomes among pregnant women and flu shots.
- Case-Control Study of Inactivated Influenza Vaccine and Spontaneous Abortion in the Vaccine Safety Datalink, 2012-13, 2013-14, and 2014-15 – During the February 2019 meeting of the Advisory Committee on Immunization Practices (ACIP), the results of a recent study of flu vaccination of pregnant women were presented by James Donahue PhD, DVM, senior epidemiologist with the Marshfield Clinic Research Institute. The study, which specifically looked at flu vaccine and miscarriage, was a matched case-control study of women ages 18-44 in the 2012-’13, 2013-’14 and 2014-’15 flu seasons. The study’s findings showed no link between flu vaccine during pregnancy and spontaneous abortion within 28 days, and supports the safety of flu vaccine in early pregnancy.
- Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009 – American Journal of Obstetrics and Gynecology, February 2011 – This article is a review of reports to the Vaccine Adverse Reporting System (VAERS), which found no unusual or unexpected patterns of reporting for pregnancy complications or adverse fetal outcomes among pregnant women and flu shots.
- Trivalent inactivated influenza vaccine and spontaneous abortion – Obstetrics & Gynecology, January 2013 – A study using Vaccine Safety Datalink (VSD) data found no increased risk of miscarriage among pregnant women who received flu vaccines in the 2005-06 or 2006-07 flu seasons.
- Inactivated Influenza Vaccine During Pregnancy and Risks for Adverse Obstetric Events – Obstetrics & Gynecology, September 2013 – A large study using VSD data found no increased risk for adverse obstetric events (like chorioamnionitis, pre-eclampsia, or gestational hypertension) for pregnant women who received the flu vaccine from 2002 to 2009 when compared to pregnant woman who were not vaccinated.
- Maternal Influenza Vaccine and Risks for Preterm or Small for Gestational Age Birth – Pediatrics, May 2014 – A study using VSD data compared pregnant women who received the flu shot with an equal number of pregnant women who did not receive the flu shot during the 2004-05 and 2008-09 flu seasons. The study found no differences between the two groups in the rates of premature delivery or small for gestational age infants.
- First Trimester Influenza Vaccination and Risks for Major Structural Birth Defects in Offspring – Pediatrics, August 2017 – A large study using VSD data found that the babies of women who received the flu shot during their first trimester had no increased risk of having children with major birth defects.
More Information
Current Flu Season (VYF)
Quick Facts About Vaccines in Pregnancy (SMFM – highriskpregnancyinfo.org)
Flu shots have been safely given to millions of pregnant people over many years.
The CDC monitors flu vaccine safety in pregnant women during each flu season using the Vaccine Adverse Event Reporting System (VAERS) – a U.S. system that monitors health concerns following vaccination. In addition, vaccine safety research is done through the Vaccine Safety Datalink (VSD). which is a collaboration between CDC and nine healthcare organizations.
View the large number of vaccine safety studies on flu vaccination during pregnancy.
The CDC, the American Academy of Family Physicians (AAFP), the American College of Nurse-Midwives (ACNM), the American College of Obstetricians and Gynecologists(ACOG), the Association of Women’s Health, Obstetric and Neonatal Nurses (AWHONN), and other medical/pregnancy experts strongly recommend that pregnant individuals get a flu shot for the best protection – for them and their babies – against serious flu illness and flu-related complications.
More Information
Current Flu Season (VYF)
Quick Facts About Vaccines in Pregnancy (SMFM – highriskpregnancyinfo.org)
Yes. Tdap vaccinations during pregnancy have been studied for both safety and effectiveness. Medical and public health experts agree that the benefits of Tdap vaccination during pregnancy outweighs any potential risks to moms and babies. See the research that has been done to make sure the Tdap vaccine during pregnancy is safe and effective for women and their babies.
The CDC, the American Academy of Family Physicians, the American College of Nurse-Midwives, the American College of Obstetricians and Gynecologists; and Association of Women’s Health, Obstetric and Neonatal Nurses all strongly recommend that women get a Tdap vaccination during the third trimester of every pregnancy to protect them and their babies against whooping cough.
No. The way that flu vaccines are made they cannot cause the flu. Flu shots are made from either flu viruses that have been ‘inactivated’ (killed) OR from proteins of a flu virus (instead of the full virus) so they can create an immune response without causing a flu infection.
While some people may get mild side effects from the flu shot like a sore arm, a headache, muscle aches or a low fever, those side effects usually begin soon after the shot and only last 1 -2 days.
Learn more about the current flu season and the flu vaccine.
Learn more about how vaccines work.
Getting a Tdap shot during every pregnancy – as opposed to before or after – allows your body to pass on some protective antibodies against whooping cough (immunity) to your baby. This is especially important since babies don’t start their own whooping cough vaccination series (DTaP) until they are 2 months old.
Getting vaccinated with Tdap during pregnancy also protects you during delivery and will make you less likely to pass whooping cough to your newborn.
A study published in Pediatrics in May 2017 looked to see how effective the Tdap vaccine was at preventing whooping cough in babies whose mothers got the vaccine while pregnant or in the hospital after giving birth. The study found that getting Tdap between the 27th through 36th weeks of pregnancy is 85% more effective at preventing whooping cough in babies younger than 2 months old.
Additionally, authors of the study, Sources of Infant Pertussis Infection in the United States published in October 2015 in Pediatrics, state that vaccinating pregnant women with Tdap increases protection of their babies.
Yes. You can safely get the flu and the Tdap (whooping cough) vaccines at the same time.
- Safety of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis and Influenza Vaccinations in Pregnancy –Obstet Gynecol, November 2015
Do I need another Tdap (whooping cough) vaccine if I already received a Tdap vaccine or tetanus vaccine (Td)?
Yes. Pregnant women can safely get the Tdap vaccine even if they recently got a tetanus-containing vaccine (Td or Tdap).
It does not matter when you got your last tetanus shot (Tdap or Td vaccine), you still need the Tdap vaccine during the 3rd trimester of each pregnancy to protect yourself and your newborn from whooping cough.
If I get the flu and Tdap (whooping cough) vaccines during my pregnancy, why does my baby need these vaccines too?
The protection (antibodies) that you pass on to your baby before birth is very important and will give them some early protection against flu and whooping cough.
However, these antibodies will only give your baby short-term protection. That’s why it is also very important for your baby to get vaccinated according to the CDC’s recommended childhood immunization schedule, so he can start building his own protection against these dangerous diseases.
You may have heard that vaccines contain all types of crazy ingredients that sound as though they don’t belong in a medical product. The truth is that a very small group of very vocal, but misinformed, individuals have made false claims regarding the safety of vaccines and their ingredients. In most instances these claims are just wrong. In other cases, the claims are from information taken out of context or are trying to purposely mislead people.
The main ingredients in vaccines are antigens, which are small amounts of the bacteria or virus against which the person is being vaccinated. Antigens are the parts of the vaccine that encourage your immune system to create antibodies to fight against future infections. To make sure that the vaccines cannot cause the disease you are trying to protect against, the antigens are altered or weakened. Learn more about how vaccines are made and how they work.
Like many of the foods we eat and beverages we drink, vaccines also contain a small amount of additional ingredients, and each has a specific, necessary function. These ingredients may be added to the vaccine to make it more effective, sterile and/or safe. These additional ingredients have been studied and are safe for humans in the amount used in vaccines.
In fact, the amount of these additional ingredients in vaccines is much less than children encounter in their environment, food and water. As the saying goes, “the dose makes the poison.” In other words, any chemical – even water or oxygen – can be toxic or even deadly in large enough quantities.
Sometimes a child may be sensitive to one of the components of a vaccine, and an allergic reaction may result. For this reason, you should discuss any allergies your child may have with their healthcare provider. Click here and see below to learn about the ingredients that may be found in certain vaccines and their purpose.
Watch These Other Short Videos from Vaccinate Your Family
Is there mercury (thimerosal) in vaccines? Is that dangerous?
I’ve heard there are ingredients in vaccines that can harm children. Is this true?
Visit VYF’s Questions about Vaccines section for answers to your questions about vaccine safety, vaccine ingredients, vaccine schedules, COVID-19 vaccines and much more.
You can find more information on flu and Tdap vaccines during pregnancy here.
You can find more information on vaccines for babies and children here.


